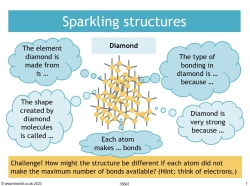
Giant covalent structures – diamond and graphite
A complete lesson for AQA GCSE chemistry (and combined science, Trilogy or Synergy) on the giant covalent substances diamond and graphite.
Students will learn about the different arrangements of carbon atoms in the two substances and the difference in the number of atoms forming the bond. They will see how the structures of diamond and graphite relate to their physical properties: how the large number of strong covalent bonds in diamond makes it very hard and gives it a high melting point, while the weak intermolecular forces in graphite make it brittle and the free electrons make the substance a conductor.
Students will also consider how covalent bonding is different from ionic bonding and have the opportunity to answer a stretch and challenge question on another giant structure, silicon dioxide.
An extract from this resource:
Graphite is also a giant covalent structure.
Covalent bonds join each carbon atom to its three nearest neighbours. The three bonds are evenly spaced in 2D, making flat sheets of hexagons (a planar molecule).
As with diamond, this repeats until there are billions of atoms bonded in this structure.
