How temperature affects the rate of reaction
This worksheet on how temperature affects the rate of reaction consists of a text containing deliberate mistakes for students to identify and correct.
There are four options: one for higher achievers (spot the errors), a second for middle achievers (spot the errors), a third for lower achievers (spot the errors, with a word bank) and a fourth for SEN or EAL students (fill in the gaps). This activity is best completed after modelling how temperature affects the rate of reaction or after students have completed the practical. However, it can also be used as a stand-alone lesson for cover. The activity should take between 10 and 15 minutes depending on the ability of the class.
This worksheet was written to be used with year 10/11 students studying for AQA Trilogy, but it may also be suitable for other exam boards and specifications.
An extract from the answers to version 1 of the text:
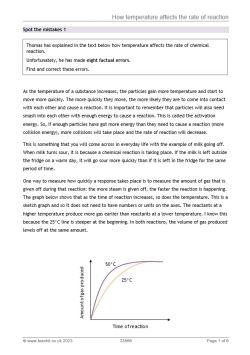
As the temperature of a substance increases, the particles gain more energy and start to move more quickly. The more quickly they move, the more likely they are to come into contact with each other and cause a reaction. It is important to remember that particles will also need smash into each other with enough energy to cause a reaction. This is called the activation energy. So, if enough particles have got more energy than they need to cause a reaction (more activation energy), more collisions will take place and the rate of reaction will increase.
