Comparing atoms
This quick comparison of atoms in terms of their size, mass and reactivity makes a great starter or plenary for the AQA GCSE chemistry or combined science module on atomic structure and the periodic table.
Students identify which statements comparing magnesium atoms and calcium ions are correct and then add information to the statement about the relationship between reactivity and losing electrons.
Answers are provided at the end of the worksheet and on the PowerPoint slides.
An extract from this resource:
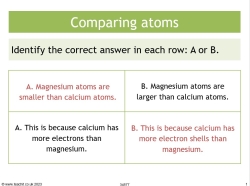
Circle the correct answer in each row.
|
Magnesium atoms are smaller than calcium atoms. |
Magnesium atoms are larger than calcium atoms. |
|
This is because calcium has more electrons than magnesium. |
This is because calcium has more electron shells than magnesium. |
