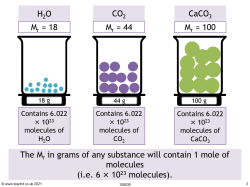
The mole – Avogadro's constant
Last updated: 15/11/2023
Contributor: Russ Church
A short but helpful PowerPoint which defines Avogadro's constant for students and includes a helpful visual summary.
There are also five calculations with moles for students to complete (with answers).
The Avogadro constant: 1 mole = 6.022 × 1023 molecules.
Example calculations from the teaching resource:
Q1. How many moles are there in 365 g of HCl?
Q2. How many moles are there in 42.5 g of NH3?
Q3. How many moles are there in 285 g of MgCl2?
All reviews
There are no reviews yet. Have you used this resource?Review this resource