Titrations exam practice
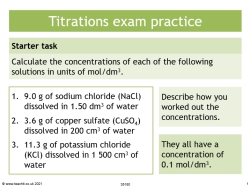
A step-by-step PowerPoint guide to determining the concentration of a reacting solution from the reacting volumes and the concentration of solutes.
Includes practice exam-style questions in the Word document along with worked answers and Higher tier exam equations in the PowerPoint.
Includes guidance for students studying GCSE Combined Science and GCSE Chemistry students.
The classroom presentation includes a starter activity and a plenary as well as a detailed explanation which takes students step-by-step through the process of how to approach exam questions.
Example question from the accompanying Word worksheet with practice questions:
A solution of sulfuric acid (H2SO4) of unknown concentration was titrated against 25.0 cm3 of a 0.105 mol/dm3 solution of sodium hydroxide. The results of the analysis are shown in the table. Calculate the concentration of sulfuric acid.
|
|
Trial |
Run 1 |
Run 2 |
Run 3 |
|
Final burette reading (cm3) |
22.55 |
40.60 |
39.30 |
38.40 |
|
Initial burette reading (cm3) |
2.05 |
21.55 |
0.40 |
19.30 |
|
Titre (cm3) |
20.50 |
19.05 |
18.90 |
19.10 |
